Oct 01, 18 · A characterization of solid lipid nanoparticles (SLN) produced starting from an emulsion prepared in acidic or neutral medium and based on Gelucire ® 50/13 as lipid phase was performed by XRay Photoelectron Spectroscopy Analysis (XPS) The smallest particle size and the highest peptide content were observed for GSHSLN produced in acidic medium GSHSLN(HAc)Gelucire® 50/13, Gattefosse) may serve as both solidifying and emulsifying agents for triglycerides The excipient is primarily a mixture of PEG 1500 mono and diesters with palmitic (C 16) and stearic (C 18) acid with an HLB value of ~13 (13) It was used for the development of solid dispersion hydrophobicJun 30, 19 · Gelucire® 50/13;

Vibrational Behavior Of Gelucire 50 13 By Raman And Ir Spectroscopies A Focus On The 1800 1000 Cm 1 Spectral Range According To Temperature And Degree Of Hydration Sciencedirect
Gelucire 50/13 gattefosse
Gelucire 50/13 gattefosse-An oral pharmaceutical composition of isotretinoin containing at least two lipidic excipients, one of them being hydrophilic (ie having an HLB value superiorJun 18, 18 · The final product is either a pure PEG ester (Gelucire® 48/16) or a mixture of PEG esters and mono, di and triglycerides (Gelucire® 44/14 and Gelucire® 50/13) The fatty acid repartition depends on the original raw material Gelucire® 48/16 and Gelucire® 50/13 are composed mainly of stearic and palmitic acids, whereas lauric acid is the



Utilizing Pluronic F 127 And Gelucire 50 13 Solid Dispersions For Enhanced Skin Delivery Of Flufenamic Acid Shazly 12 Drug Development Research Wiley Online Library
Jan 01, 17 · The lipid components Gelucire® 50/13 (stearoyl macrogolglycerides, GATTEFOSSÉ GmbH, Bad Krozingen, Germany), Witepsol® S55 (solid triglycerides containing hydrogenated cocoglycerides, beeswax and ceteareth25, CREMER OLEO GmbH & Co KG, Hamburg, Germany), and Capryol® 90 (propylene glycol monocaprylate, Gattefossé GmbH, Bad KrozingenGelucire 44/14 Acylglycerol fraction PEG ester fraction Figure 1 Thermograms of the first melting of Gelucire® 44/14 (solid line), the acylglycerol fraction of Gelucire® 44/14 (dotted line), and the PEG ester fraction of Gelucire® 44/14 (dashed dot dot line) at a heating rate of 3 °C/min 268 OCL VOL 16 N° 4 JUILLETDÉCEMBRE 09Jun 01, 18 · The following are theexamples of hydrophilic grades of gelucire such as 50/13, 44/14, 48/16, 55/18, 35/10, 48/09 Among the above grades, gelucire 50/13 and 44/14 were selected for review as a lot of research works are published using these two grades 12 Gelucire ®
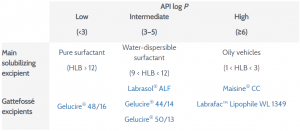
Gelucire 50/13 product page Gattefosse Published 10 Shohin et al, (Journal of Pharmaceutical Sciences Vol 103 pages Published February 14;Application Water dispersible surfactant, Solubilizer, Bioavailability enhancer, Component of SELF, Matrix for modified release, Multiparticulates;Functionality Solubilizer for poorlysoluble APIs and bioavailability enhancer Single excipient formulation system selfemulsifies in aqueous fluid
Provider of personal care ingredients and pharmaceutical excipients, Gattefossé has been exploring the very best of nature and science since 10Gelucire® 50/13 (stearoyl polyoxyl32 glycerides) is a nonionic waterdispersible surfactant for lipidbased formulations to solubilize and increase oral bioavailability of poorly watersoluble APIs Selfemulsifies in aqueous media forming a fine dispersion, ie, microemulsion (SMEDDS)Detailed Specifications for Labrafac™ PG from Gattefossé Product Specs;



Effect Of Different Lipids And Surfactants On Formulation Of Solid Lipid Nanoparticles Incorporating Tamoxifen Citrate Topic Of Research Paper In Nano Technology Download Scholarly Article Pdf And Read For Free On Cyberleninka


Pdf Gelucire 44 14 Based Immediate Release Formulations For Poorly Water Soluble Drugs
Application Oily vehicle, Solubilizer, Bioavailability enhancer, Component of SELF and microemulsions;Other products like Gelucire® 50/13 and 48/16, due to their solidstate characteristics, are suitable for preparation of solid dispersions by melt extrusion or spray atomization for preparation of selfemulsifying solid dosage formsThe aim of this study was to develop a formulation containing fenofibrate and Gelucire(®) 50/13 (Gattefossé, France) in order to improve the oral bioavailability of the drug Particles from gas saturated solutions (PGSS) process was chosen for investigation as a



New Pharmacopoeia Monographs For Gattefosse Speciality Excipients



Utilizing Pluronic F 127 And Gelucire 50 13 Solid Dispersions For Enhanced Skin Delivery Of Flufenamic Acid Shazly 12 Drug Development Research Wiley Online Library
Gelucire 50/13, and Gelucire 43/01 as lipid carriers Ranitidine HCl– lipid granules were prepared by the melt granulation technique and evaluated for in vitro floating and drug release The results revealed that the moderate amount of Gelucire 43/01 and ethyl celluloseGelucire® 44/14, Gelucire® 48/16, Gelucire® 50/13 and the Labrafil® series (Table 2) consist of selfemulsifying excipients that may be in SEDDS / SMEDDS Several of these products listed are liquid or semisolid, hence suitable for soft or hard shell capsules Other products, such as Gelucire® 48/16 and Gelucire® 50/13 areLabrafil® M 1944 CS;



Journal Of Applied Pharmaceutical Science



Lauroyl Polyoxylglycerides Functionalized Coconut Oil Enhancing The Bioavailability Of Poorly Soluble Active Substances Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For Free On Cyberleninka Open
Functionality Oily phase for topical formulations Oily vehicle and solubilizer for solutionsThe aim of this study was to develop a formulation containing fenofibrate and Gelucire(®) 50/13 (Gattefossé, France) in order to improve the oral bioavailability of the drug Particles from gas saturated solutions (PGSS) process was chosen for investigation as a manufacturing process for producing a solid dispersionAbstract, page 267, page ) Elmalehm H el al, Probe into the physical properties of a Gelucire 44/14 pharmaceuitcal formulation Published online 14



Gelucire A Versatile Polymer For Modified Release Drug Delivery System Sciencedirect



Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery
Gelucire® 44/14 Gelucire® 48/16 Gelucire® 50/13 Labrafac™ lipophile WL 1349 Labrafac™ PG Labrafil® M 1944 CS Labrafil® M 2125 CS Labrafil® M 2130 CS Labrasol® ALF Lauroglycol™ 90 Lauroglycol™ FCC Maisine® CCLabrasol®, Gelucire® 44/14, Gelucire® 48/16, Gelucire® 50/13 and the Labrafil® series (Table 2) consist of selfemulsifying excipients that may be in SEDDS / SMEDDS Several of these products listed are liquid or semisolid, hence suitable for soft or hard shell capsulesBetween gelucire 50/13 and poloxamer 1 and to formulate the optimized solid dispersion into immediate release tablets MATERIALS and METHODS Materials Bosentan was received as gift sample from MSN Pharma, India Gelucire 50/13 was received as gift sample from Gattefosse, India, Poloxamer 1 was procured from Sigma Aldrich, India



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents
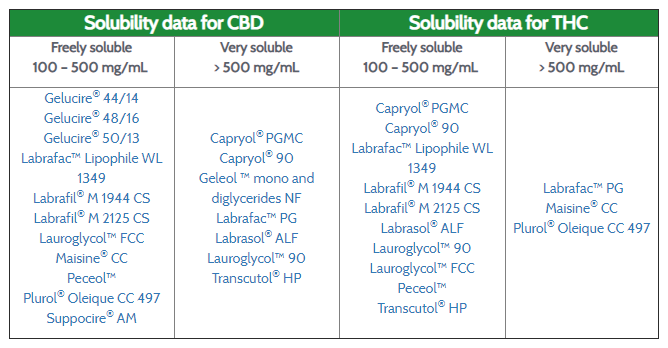


Formulating Cannabinoids Pharma Excipients
Generic filters Hidden label Hidden labelGelucire 50/13 in a ratio of 1 to 175 (drug Gelucire) achieved a drug release of % in 4 hours, a 5fold increase compared to pure carvedilol When incorporating 10% Dαtocopheryl polyethylene glycol succinate (vitamin E TPGS/ TPGS) a higher drug release was observed (%) Parallel artificial membrane permeability assay was used toMar 01, 19 · Gelucire® 50/13 (Gattefossé) Lipids can affect biopharmaceutical properties of the drugs leading to improved drug solubility in the intestinal fluid, protection of the drug against enzymatic degradation as well as the formation of lipoproteins promoting the lymphatic transport of highly lipophilic drugs 10 , 21



Gelucire 48 16 From Gattefosse Product Description And Details



Thermal And Fractal Analysis Of Diclofenac Gelucire 50 13 Microparticles Obtained By Ultrasound Assisted Atomization Journal Of Pharmaceutical Sciences
USB2 US11/2,363 USA USB2 US B2 US B2 US B2 US A US A US A US B2 US B2 US B2 Authority US United States Prior art keywords composition isotretinoin skin disorder fatty acid acid esters Prior art date Legal status (The legal status is an assumptionGelucire 50/13 (HPMCHPCGel 50/13) were prepared by the same method stated above in the ratio of 111 In this case, the weighed samples of HPMC and HPC were dissolved in 2 ml of absolute ethanol while the Gelucire 50/13 was dissolved in 2 ml of methylene chloride 23 Preparation of the IndHPMCGel 50/13 and IndHPMCHPCGelGelucire® 50/13 A nonionic waterdispersible surfactant for lipidbased formulations to solubilize and increase oral bioavailability of poorly watersoluble APIs Selfemulsifies in aqueous media forming a fine dispersion, ie, microemulsion (SMEDDS)



Improvement In The Dissolution Rate And Tableting Properties Of Cefuroxime Axetil By Melt Granulated Dispersion And Surface Adsorption Sciencedirect



Characterization Of Carbamazepine Gelucire 50 13 Microparticles Prepared By A Spray Congealing Process Using Ultrasounds Passerini 02 Journal Of Pharmaceutical Sciences Wiley Online Library
Gelucire® 50/13 A nonionic waterdispersible surfactant for lipidbased formulations to solubilize and increase oral bioavailability of poorly watersoluble APIs "Download ourJul 01, 09 · Gelucire 50/13 was provided by Gattefosse (Cedex, France) and has mp 50 °C and HLB 13 All other materials and reagents were of analytical grade of purity22 Methods221 Preparation of physical mixtureGelucire is a waxy pellet, so it is crushed to fine particles firstly to prepare the physical mixturePeceol™ Plurol® Oleique CC 497;



Pdf Improvement In The Dissolution Rate And Tableting Properties Of Cefuroxime Axetil By Melt Granulated Dispersion And Surface Adsorption Sarwar Beg Academia Edu



Pdf Gelucire A Versatile Polymer For Modified Release Drug Delivery System
Mar 18, 15 · The aim of this study was to develop a formulation containing fenofibrate and Gelucire(®) 50/13 (Gattefossé, France) in order to improve the oral bioavailability of the drug Particles from gas saturated solutions (PGSS) process was chosen for investigation as a manufacturing process for producing a solid dispersionGelucire® 50/13 Gattefossé Solubilizer for poorlysoluble APIs and bioavailability enhancer Single excipient formulation system selfemulsifies in aqueous fluid into coarse emulsion—LFCS Type III (SMEDDS) Modulation of drug release Lipid binder in melt processesMain components of Gelucire® 50/13 R=C16 or C18 Schematic structures PEG1500 esters O H R C O O 3 3 O C O R R C O O 3 3 Phospholipon® 90G Phospholipon® 90G was received as a gift sample from Phospholipid GmbH (Nattermannalle, Germany) This is a purified, deoiled, and granulated soy lecithin with a high phosphatidylcholine
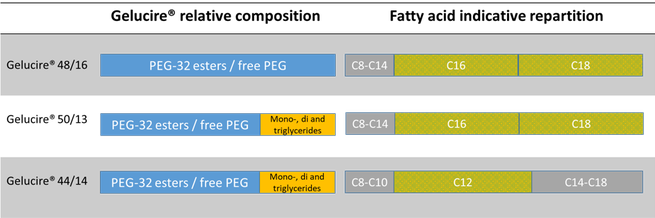


The Gelucire Family Semi Solid Excipients By Gattefosse Pharma Excipients
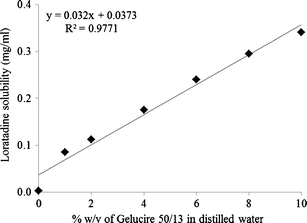


Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Springerlink
Gelucire 5013,CAS,,Gelucire 5013 suppliers Approved Bulk Manufacturers Want to be listed as an approved manufacturer (Free service but requires approvement)?An oral pharmaceutical composition of isotretinoin containing at least two lipidic excipients, one of them being hydrophilic (ie having an HLB value superiorGelucire® 50/13 Gattefossé Solubilizer for poorlysoluble APIs and bioavailability enhancer Single excipient formulation system selfemulsifies in aqueous fluid into coarse emulsion—LFCS Type III (SMEDDS) Modulation of drug release Lipid binder in melt processes


Special Feature Excipients Enhancing The New Poorly Soluble Apis



Wo 09 A1 Once A Day Formulation Of Angiotensin Receptor Blockers The Lens Free Open Patent And Scholarly Search
An oral pharmaceutical composition of isotretinoin containing at least two lipidic excipients, one of them being hydrophilic (ie having an HLB value superiorLabrafil® M 2130 CS;WOA1 PCT/BE01/ BEW WOA1 WO A1 WO A1 WO A1 BE W BE W BE W WO A1 WO A1 WO A1 Authority WO WIPO (PCT) Prior art keywords isotretinoin pharmaceutical composition mixtures fatty acid formulation Prior art date



Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems


Excipients For Solubility And Bioavailability Enhancement Pharma Excipients
Labrafac™ lipophile WL 1349;Dec 05, 07 · Gelucire® 44/14 has achieved official USPNF status with pending Food Additive (FCC) status Gelucire® 44/14 is a versatile semisolid lipidic excipient, proven to improve the bioavailability of poorly soluble drugs It has a high HLB (14) and can be used either alone or as part a self emulsifying formulation (SEDDS/SMEDDS)Gelucire® 44/14 A nonionic waterdispersible surfactant for lipidbased formulations to solubilize and increase oral bioavailability of poorly watersoluble APIs Selfemulsifies in aqueous media forming a fine dispersion, ie, microemulsion (SMEDDS)


Journal Of Applied Pharmaceutical Science


Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Nano Market
Jun , 12 · Application of Acconon C50 and Gelucire 50/13 as Both Solidifying and Emulsifying Agents for Medium Chain Triglycerides Journal of Excipients and Food Chemicals , Sl, v 3, n 2, p 92, june 12Labrafil® M 2125 CS;GELUCIRE 50/13 and GELUCIRE 53/10 can be used according to our invention, but GELUCIRE 50/13 has been found to be particularly effective It is composed of fatty acid (majority of C 16 and C 18) esters of glycerol, PEG esters and free PEG



Pdf Gelucire A Versatile Polymer For Modified Release Drug Delivery System



Excipient Guide Gattefosse
May 10, 18 · One excipient that has stimulated interest in lipidbased solid dispersion formulations is Gelucire 44/14 (Gattefosse Corp, St Priest, France) This selfemulsifying excipient that exists as a waxy semisolid at ambient room temperature is a mixture of glyceryl and PEG 1500 esters of longchain fatty acids and is listed in the European Pharmacopoeia asGelucire® 48/16, a novel carrier available in powder and pellet forms, is PEG32 stearate, while, conventional gelucires, Gelucire® 44/14 and Gelucire® 50/13 are lauroyl polyoxyl32glycerides NF and stearoyl polyoxyl32 glycerides NF respectively



Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems


Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Springerlink
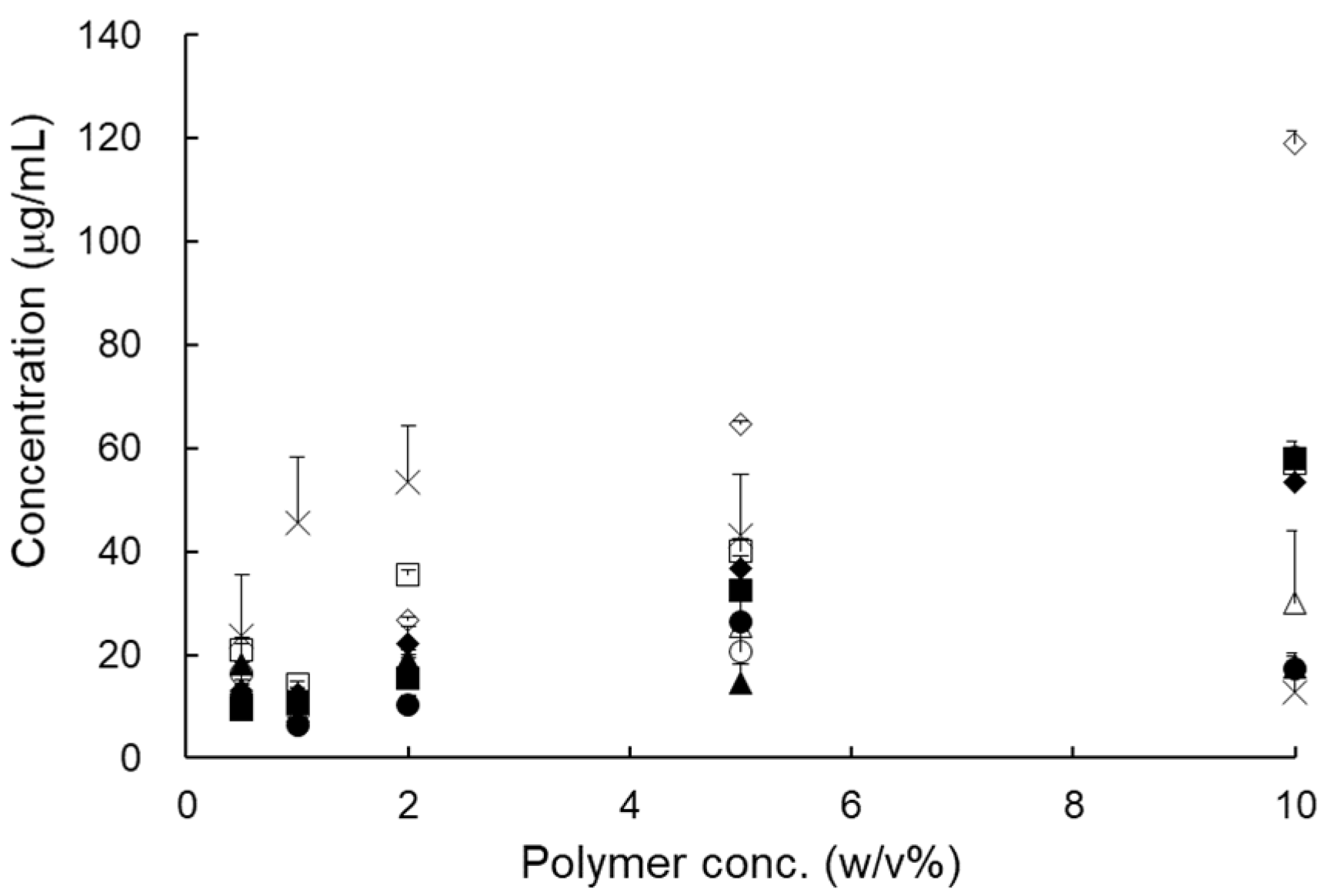


Pharmaceutics Free Full Text The Development And Optimization Of Hot Melt Extruded Amorphous Solid Dispersions Containing Rivaroxaban In Combination With Polymers Html



Design And Evaluation Of Self Emulsifying Drug Delivery Systems Sedds Of Nimodipine Abstract Europe Pmc



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents
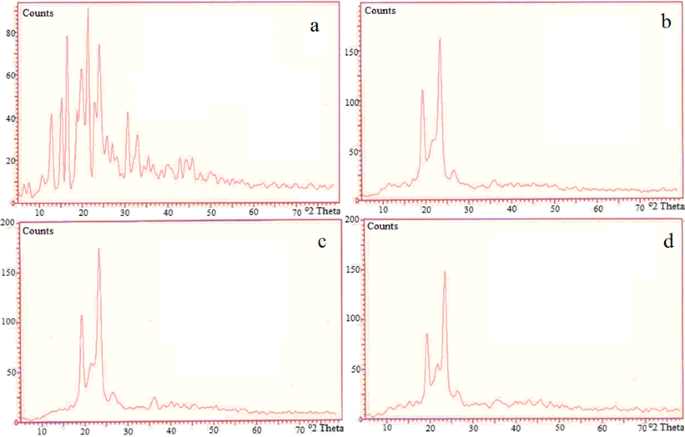


Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Springerlink



Pdf Lipid Nanocarriers Gelupearl Containing Amphiphilic Lipid Gelucire 50 13 As A Novel Stabilizer Fabrication Characterization And Evaluation For Oral Drug Delivery
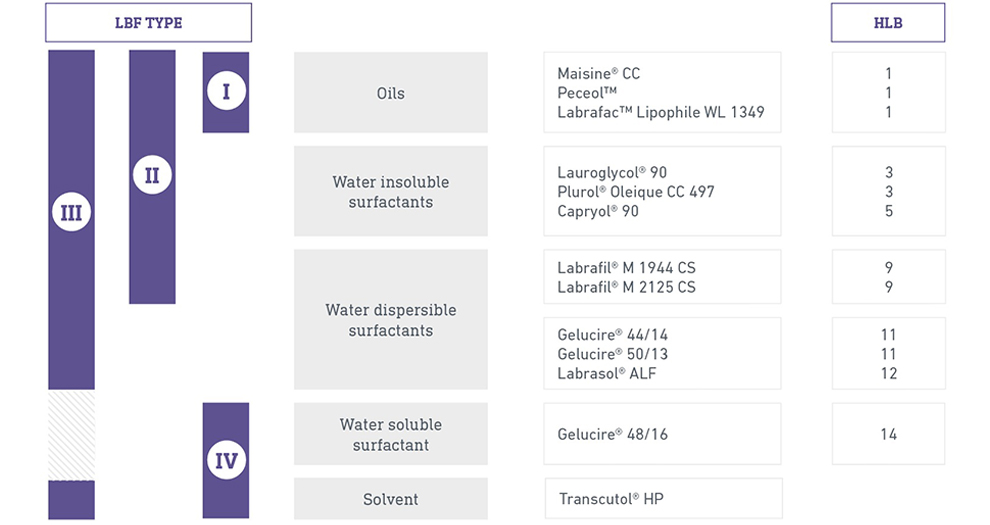


Lipid Based Formulations Winning Strategy For Oral Bioavailability Enhancement



Excipient Guide Gattefosse



Table 1 From Solubility And Dissolution Enhancement Of Gliclazide By Solid Dispersion Technique Semantic Scholar



Brochure Veterinary Medicines With Gattefosse Excipients Tablet Pharmacy Emulsion
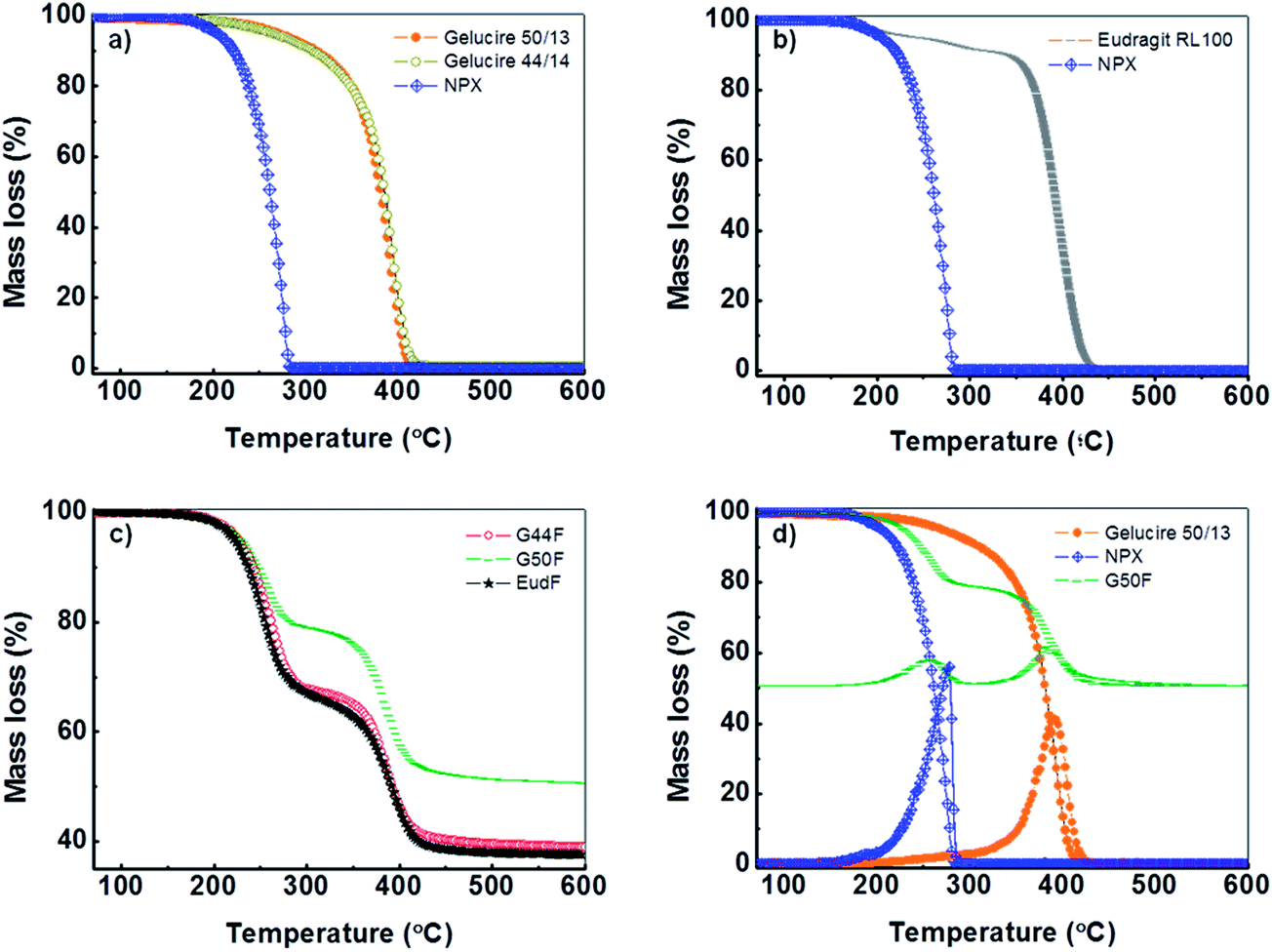


Preparation And Study Of Naproxen In Silica And Lipid Polymer Hybrid Composites Rsc Advances Rsc Publishing



Carvedilol Solid Dispersion For Enhanced Oral Bioavailability Using Rat Model



Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems



References In Gelucire Based Nanoparticles For Curcumin Targeting To Oral Mucosa Preparation Characterization And Antimicrobial Activity Assessment Journal Of Pharmaceutical Sciences



Excipient Guide Gattefosse



Gelucire 50 13 Pharma Excipients



Woa1 Composition Google Patents



Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery



Vibrational Behavior Of Gelucire 50 13 By Raman And Ir Spectroscopies A Focus On The 1800 1000 Cm 1 Spectral Range According To Temperature And Degree Of Hydration Sciencedirect


Jang Choi And Kang Preparation Of Solid Dispersion Of Everolimus In Gelucire 50 13 Using Melt Granulation Technique For Enhanced Drug Release



Lyotropic Behavior Of Gelucire 50 13 By Xrd Raman And Ir Spectroscopies According To Hydration Sciencedirect



New Pharmacopoeia Monographs For Gattefosse Speciality Excipients



Lipid Based Formulations A Winning Strategy For Oral Bioavailability Enhancement



Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems
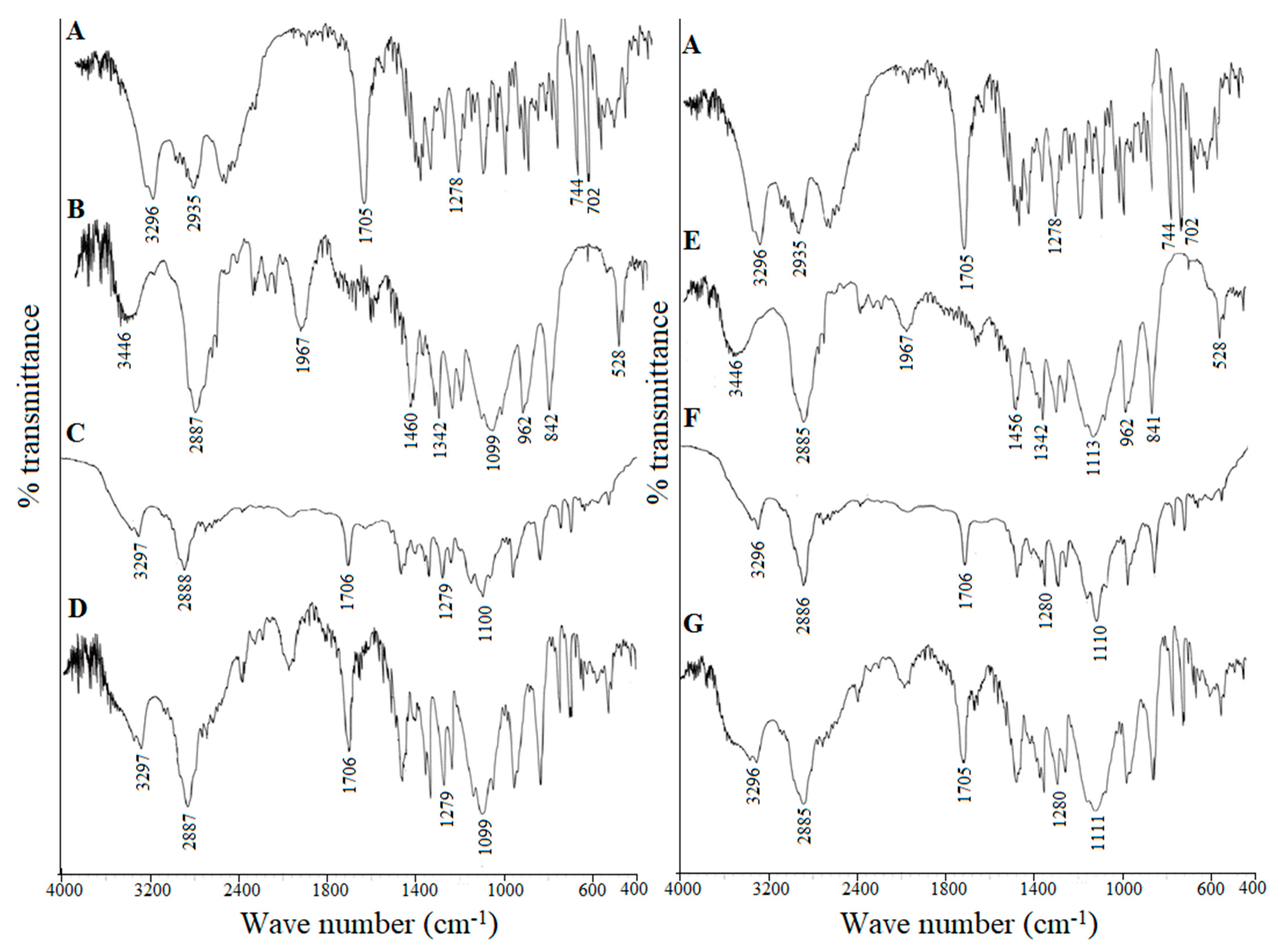


Pharmaceutics Free Full Text Improved Dissolution Rate And Intestinal Absorption Of Fexofenadine Hydrochloride By The Preparation Of Solid Dispersions In Vitro And In Situ Evaluation Html



Pdf Influence Of Various Polymers On The Improvement Of Etodolac Solubility And Dissolution Rate Via Solid Dispersion Technique Semantic Scholar



Journal Of Applied Pharmaceutical Science



Pdf Solubility And Dissolution Enhancement Of Gliclazide By Solid Dispersion Technique Ip Innovative Publication Pvt Ltd Academia Edu



Woa1 Composition Google Patents



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents
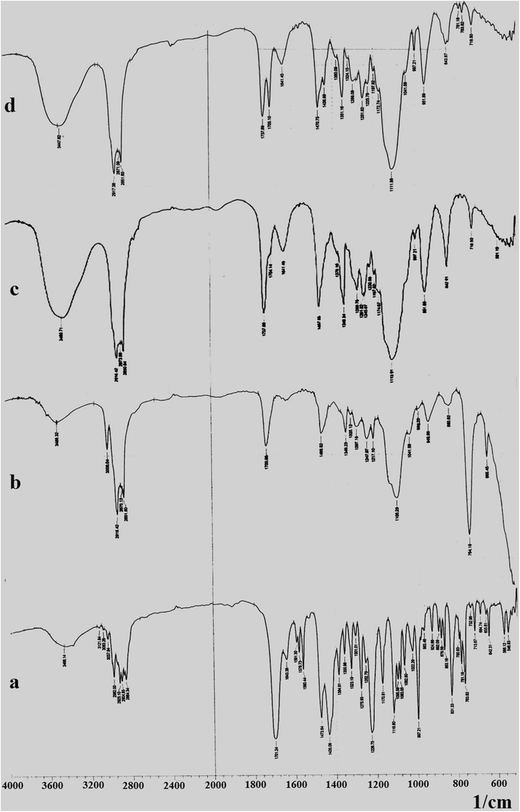


Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Springerlink



Improvement Of Solubility And Dissolution Rate Of Indomethacin By Solid Dispersions In Gelucire 50 13 And Peg4000 Abstract Europe Pmc



Gelucire Twitter Search



Oral Route Bioavailability 2i06 Pharmaceutical Formulation Emulsion



Enhancement Of Albendazole Dissolution Properties Using Solid Dispersions With Gelucire 50 13 And Peg Sciencedirect



An Investigation Into The Mechanism Of Dissolution Rate Enhancement Of Poorly Water Soluble Drugs From Spray Chilled Gelucire 50 13 Microspheres Journal Of Pharmaceutical Sciences



Carvedilol Solid Dispersion For Enhanced Oral Bioavailability Using Rat Model



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents



Woa1 Composition Google Patents
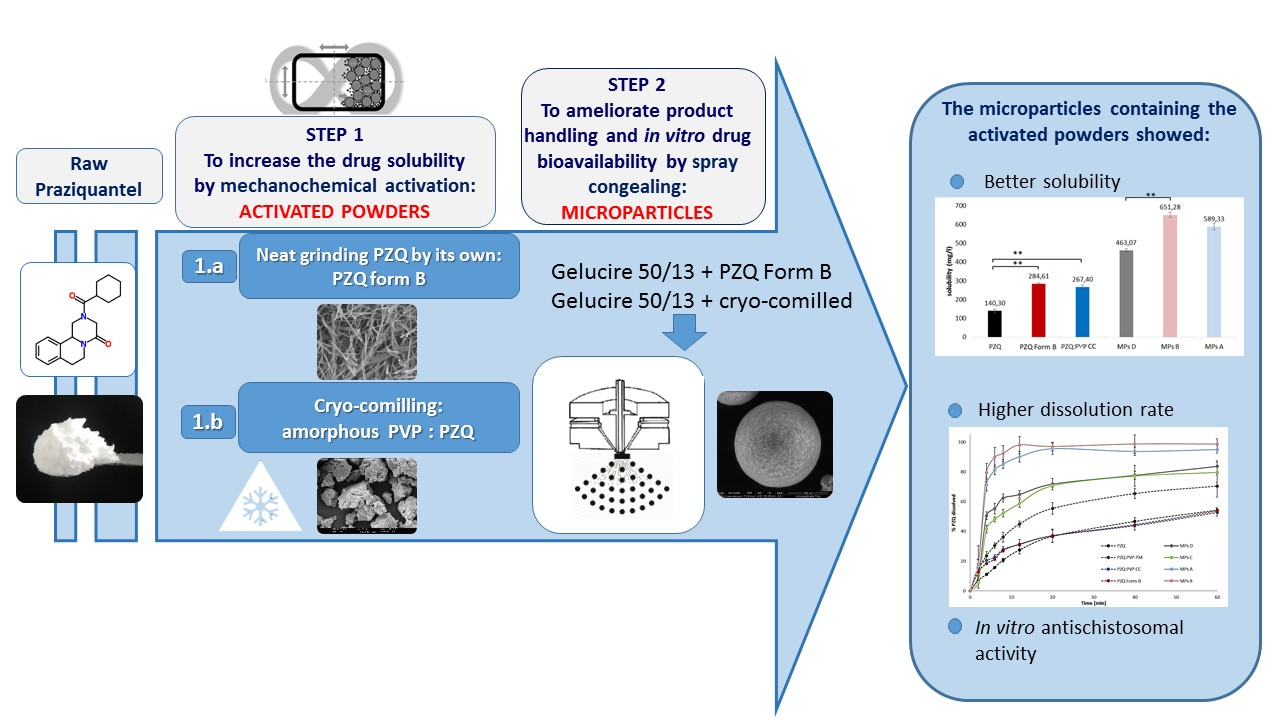


Ijms Free Full Text Combining Mechanochemistry And Spray Congealing For New Praziquantel Pediatric Formulations In Schistosomiasis Treatment Html



0 件のコメント:
コメントを投稿